-
-
ABOUT US

-

-

SERVICE
SERVICE
Cyclic peptide synthesis
1. Amide Cyclization
Structural features: A cyclic structure is formed by condensation of the side chain or terminal amino and carboxyl groups.
Head-to-tail amide cyclization: For example, the tumor-targeting peptide RGD cyclic peptide, whose structure is formed by the amide bond cyclization between the N-terminal amino group and the C-terminal carboxyl group in the polypeptide sequence. Examples include c(RGDYK) and c(RGDFK).
Side-chain amide cyclization: This is achieved by cyclization through the amino and carboxyl groups on the side chain groups in the polypeptide sequence. For example, selective protection and removal of the side chain carboxyl group of Lys (lysine), Glu (glutamic acid), or Asp (aspartic acid) can be used to achieve cyclization of the polypeptide at the precise site.
2. Disulfide Bond Cyclization
Structural features: Two cysteine residues form a -S-S- covalent bond.
Technical advantages: Redox controllable, supports double-ring/multi-ring structures.
The formation of disulfide bonds has always been a difficulty in polypeptide synthesis. Through unremitting research and accumulation, our company has already possessed quite mature multi-pair disulfide bond cyclization technology. Currently, we are able to synthesize polypeptides with three or four pairs of disulfide bonds with high success rates. Continuously overcoming experimental difficulties, constantly improving product quality, and relentlessly striving to meet customer requirements are the unchanging tenets of our company.
Successful cases:
Solid-phase synthesis sequence DC*TSHNGAC*NHHSHC*C*SNVC*NTWAHLC*T, and successful modification with three pairs of disulfide bonds.
HPLC analysis:
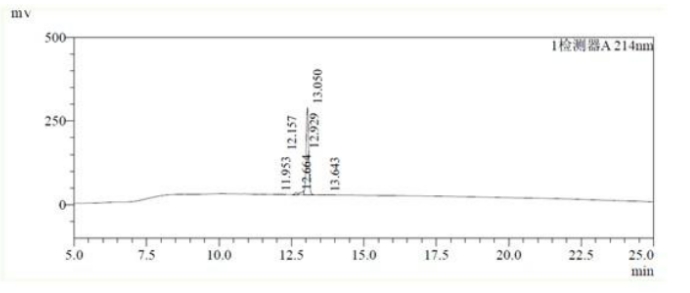
MS analysis:
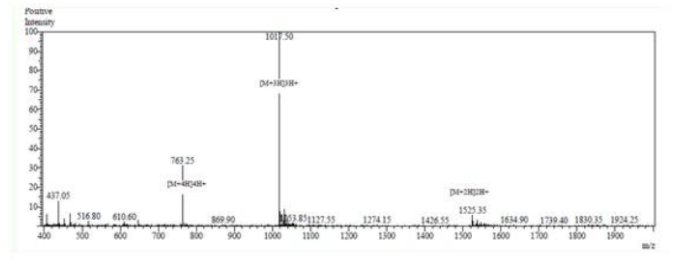
3. Stapled Peptides
Structural features: Alkene "stapling" technology stabilizes the α-helix structure.
Two amino acid residues in the peptide chain are connected by a chemical cross-linking agent. Common cross-linking methods include carbon-carbon bond cross-linking and ether bond cross-linking. For example, using alkenylated amino acid residues for cross-linking, a carbon-carbon double bond is formed under light irradiation or catalysis, connecting different parts of the peptide chain together to form a stable cyclic structure.
In addition to the cross-linking structure, stapled peptides can also modify the side chains of amino acids.
4. Thioether Cyclization
Structural features: Cysteine reacts with haloalkanes/maleimides to generate a C-S-C bond, with excellent in vivo stability and supporting site-specific cyclization.
5. Bicycle® Peptides
Structural features: Tripeptide scaffold forms a bicyclic topology.
6. Click Chemistry Cyclization
Structural features: CuAAC/SPAAC reaction constructs a triazole ring or tetrazine ring, reaction efficiency >95%, compatible with solid-phase/liquid-phase synthesis
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Address: Model B204, B205-1, Hi-tech Capital City High-end Equipment Incubation Base, No. 23, Hangbu Road, Baiyan Science Park, Hi-Tech Zone, Hefei City
Sales Tel: 19856166839; 15705607678; 17354056623
Fax: 0551-62869631
E-mail: sales@bankpeptide.com





